Anti-PD1 Immunotherapy in Hodgkin's Lymphoma: Clinical Trial Results (Dec 2014)
 Anti-PD1 treatments are showing tremendous results for Hodgkin's Lymphoma patients who have not responded to conventional treatments.
Anti-PD1 treatments are showing tremendous results for Hodgkin's Lymphoma patients who have not responded to conventional treatments.The results are coming out at the American Society of Hematology (ASH) conference, December 2014 and creating great excitement.
Here are summaries on 2 clinical trials of anti-PD1 in Hodgkin's Lymphoma patients that failed previous treatments.
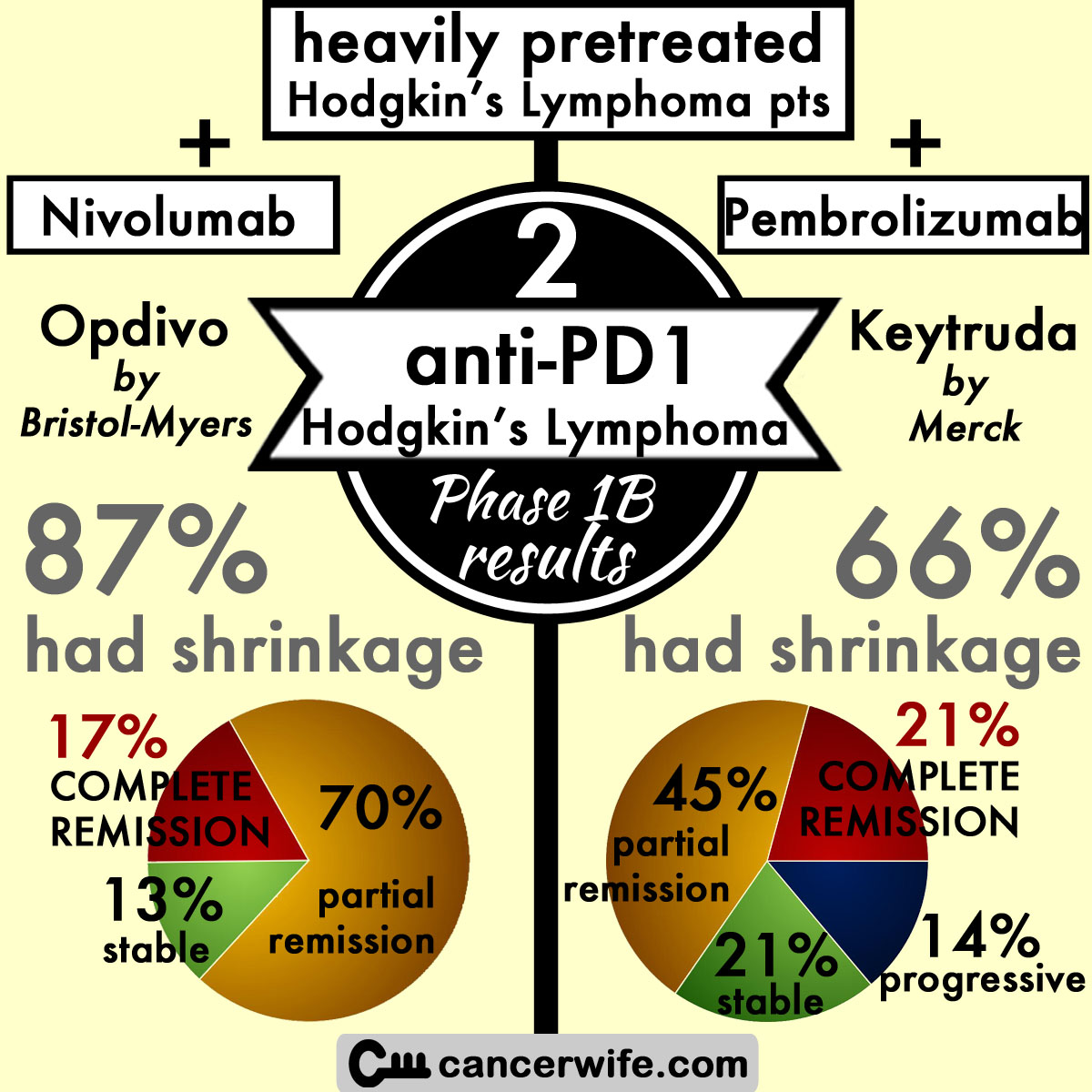
Ongoing Phase Ib Clinical Trial Results of anti-PD1 (Bristol Myer's Opdivo, nivolumab) for Hodgkin’s Lymphoma
Video of Mayo Clinic Doctor explaining anti-PD1 and results of this trial (News article on video)
Summary of press release by Bristol Myers:
What:
Opdivo (anti-PD1 by Bristol Myers, nivolumab) every 2 weeks until disease progression or complete response or for a maximum of 2 years, maximum dose 3mg/kg in CheckMate-039 clinical trial.
Who:
23 patients with relapsed or refractory Hodgkin's lymphoma
Results:
overall response (shrinkage seen) = 87% (n=20) --> 1st response within 8 weeks = 60% (n=12) (range of response: 3-39 weeks)
complete remission = 17% (n=4)
partial response = 70% (n=16)
stable disease = 13% (n=3)
—> All the patients had a tumor controlled by anti-PD1 at one point.
progression free survival at 24 weeks = 86%
Common side effects: rash (22%) and decreased platelets (17%). No treatment-related Grade 4 or 5 adverse events.
Where are the patients now?
Out of 23 patients, 11 patients still on study (48%), 6 patients discontinued due to stem-cell transplantation (26%), 4 patients discontinued due to disease progression (17%), 2 patients discontinued due to drug toxicity (9%).
Ongoing Phase 1b Clinical Trial Results of anti-PD1 (Merck's Keytruda, pembrolizumab) for Hodgkin’s Lymphoma
Summary of press release by Merck:
What:
Keytruda (anti-PD1 by Merck, pembrolizumab) in KEYNOTE-013 clinical trial.
Who:
29 patients with relapsed or refractory classical Hodgkin's lymphoma
Results:
overall response (shrinkage seen) = 66% (n=19) —> median response at 12 weeks, median duration of response not yet reached (range 1+ to 185+ days)
complete remission = 21% (n=6)
partial response = 45% (n=13)
stable disease = 21% (n=6)
—> 86% (n=25) of the patients had a tumor/ tumors controlled by anti-PD1 at one point.
progressive disease = 14% (n=4)
Common side effects: hypothyroidism (n=3), pneumonitis (n=3), constipation (n=2), diarrhea (n=2), nausea (n=2), hypercholesterolemia (n=2), hypertriglyceridemia (n=2) and hematuria (n=2).
55% patients experienced at least one treatment-related side event. No Grade 4 treatment related side effect or death reported.



