Anti-PD1 Immunotherapy in Hodgkin's Lymphoma: Why It Works and Future Directions.
 There have been remarkable results of anti-PD1 causing remissions in heavily pretreated Hodgkin's Lymphoma patients.
There have been remarkable results of anti-PD1 causing remissions in heavily pretreated Hodgkin's Lymphoma patients.
Here we examine why this cancer immunotherapy can change the way Hodgkin's Lymphoma patients are treated in the future.
Hodgkin's Lymphoma is a good target for anti-PD1.
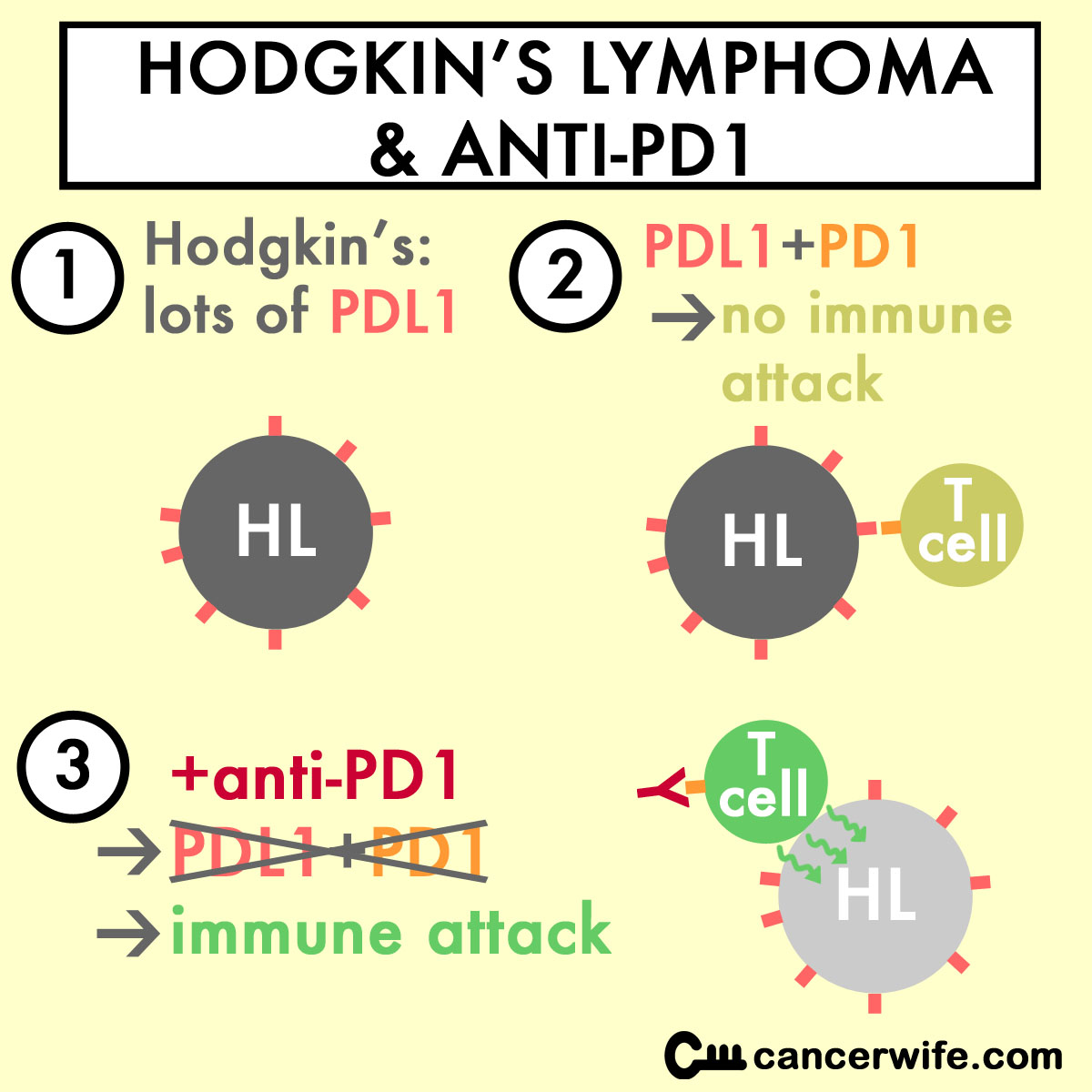
The Epstein-Barr Virus (EBV) and translocations (at location 9p24) in Hodgkin's Lymphoma (HL) cells cause high amounts of PD-L1 to be made in HL cells (Green MR et al, 2012). The abundance of PD-L1 on tumor cells shut down the T cells' ability to kill the tumor cells.
Anti-PD1 will block the "immune shield" PD-L1 confers on HL cells, allowing the T cells to kill the HL cells.
From a NYTimes article on anti-PD1 and HL, "Hodgkin’s lymphoma was thought to be particularly susceptible to these types of drugs because its cells have a genetic abnormality that leads to a production of a large amount of PD-L1."
anti-PD1 is even able to ellicit tumor shrinkage in hematological malignancies refractory to allogenic stem cell transplants.
This paper showed that leukemias refractory to allogenic stem cell transplants are high in PDL1 (ligand of PD1) and the T cells in those patients have increased PD1 on T cells, leading to the T cells not attacking the leukemia (Norde WJ, et al. Cancer Res. 2011 Aug 1;71(15):5111-22.)
"Taken together, our findings indicate that the PD-1/PD-L pathway can be hijacked as an immune escape mechanism in hematological malignancies. Furthermore, they suggest that blocking the PD-1 immune checkpoint offers an appealing immunotherapeutic strategy following alloSCT in patients with recurrent or relapsed disease."
Anti-PD1 will become the foundation for Hodgkin's Lymphoma treatment.
In this article about anti-PD1 and HL:
”Furthermore, the results are "particularly exciting" in "suggesting a future in which anti-PD-1 therapy will become the foundation for the treatment of Hodgkin's Lymphoma, possibly sparing patients both short- and long-term toxic effects of combination chemotherapy," they said.
"The results are extremely encouraging," said senior study author Philippe Armand, MD, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, in an interview with Medscape Medical News.
"We get excited when we see a 30% response rate in this population," he added.
On the basis of these results, the US Food and Drug Administration has granted nivolumab a breakthrough therapy designation in relapsed HL, and a phase 2 study is under way."



